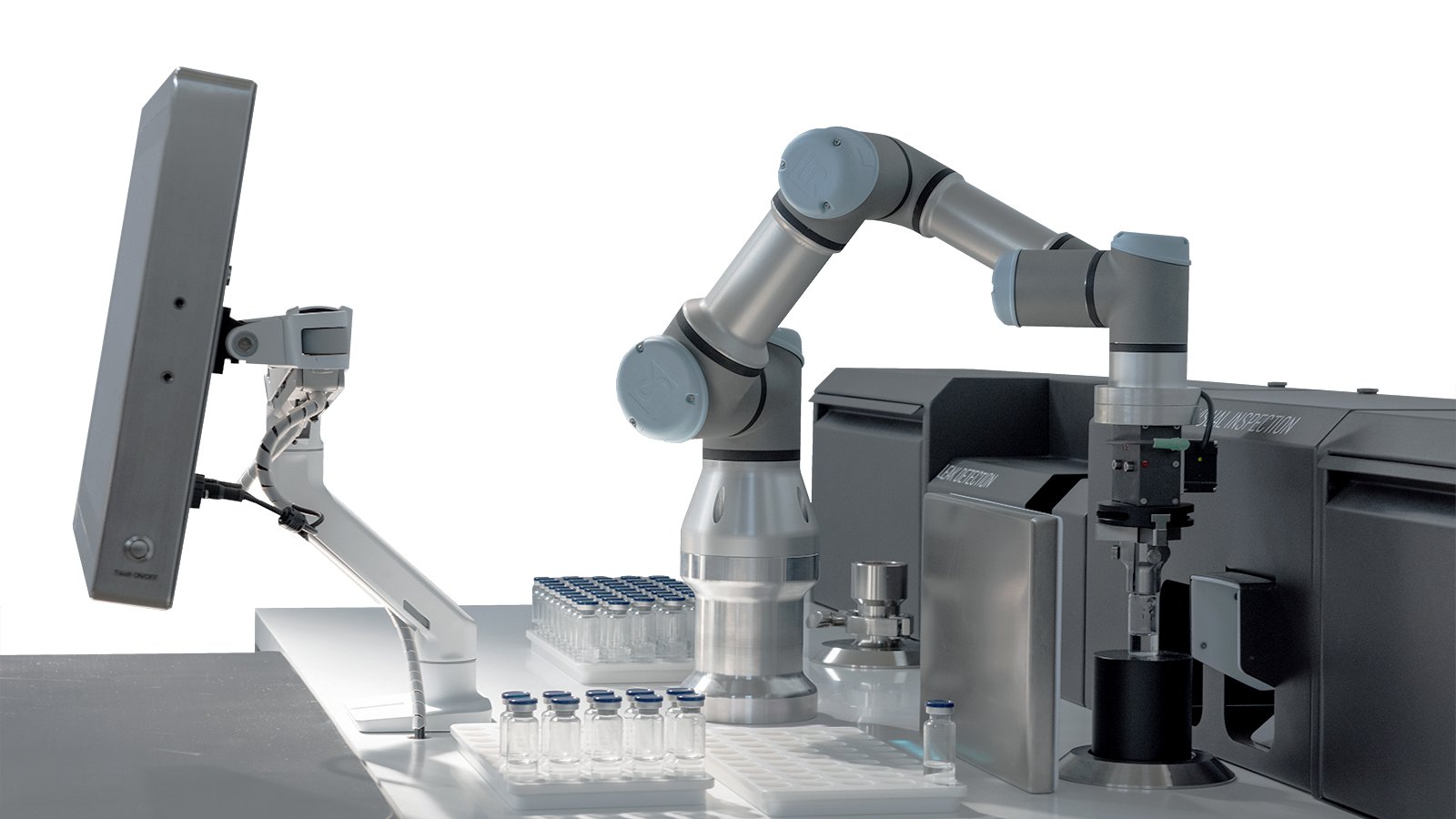
SAIL Smart Automated Inspection Laboratory
Pharmaceuticals
SAIL is an automated inspection laboratory for different container types, sizes, and contents bringing together CCIT, AVI and HGA all in one machine suitable for in-process, clinical trials and laboratory use.
PLAY VIDEO
Ampoules
BFS
Carpoules
Cartridges
Prefilled Syringes
Vials
Highlights
- All in one Industry 4.0 based laboratory
- Ergonomic workstation
- Scientific and objective inspection
- 100% traceability
- Time & cost saving