PK-VIS In-line Automatic Visual Inspection Tester
Pharmaceuticals
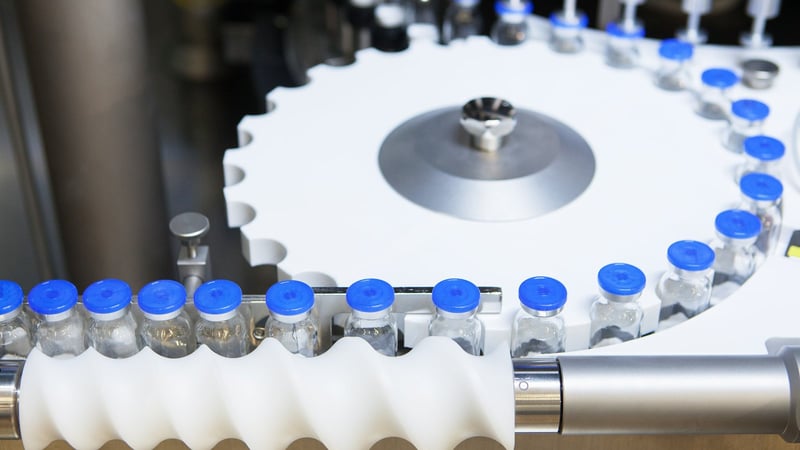
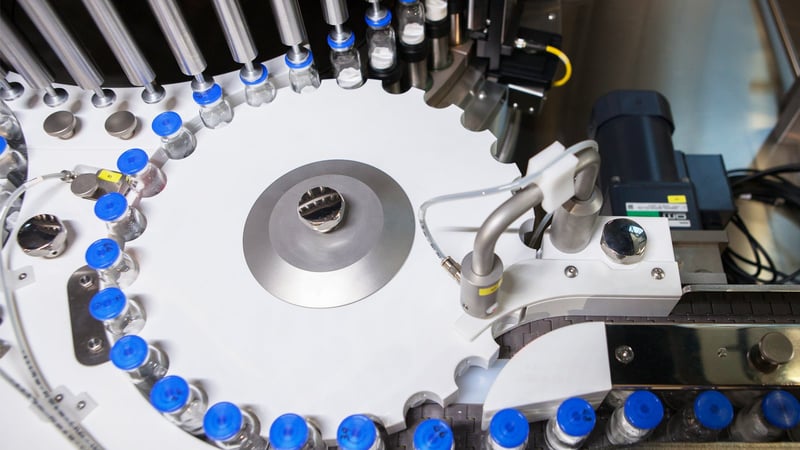
In-line and Off-line Non-Invasive, Non-Destructive Automatic Visual Inspection (AVI) System of containers with pharmaceutical product at high production speed
PLAY VIDEO
Ampoules
BFS
Bottles
Carpoules
Vials
Highlights
- High inspection solutions flexibility
- High stability of application system and validation principles
- Hardware and Software optimization according to customer URS
- AVI no limited visual defects detection
- CFR part 11 compliance and 4.0 full integration