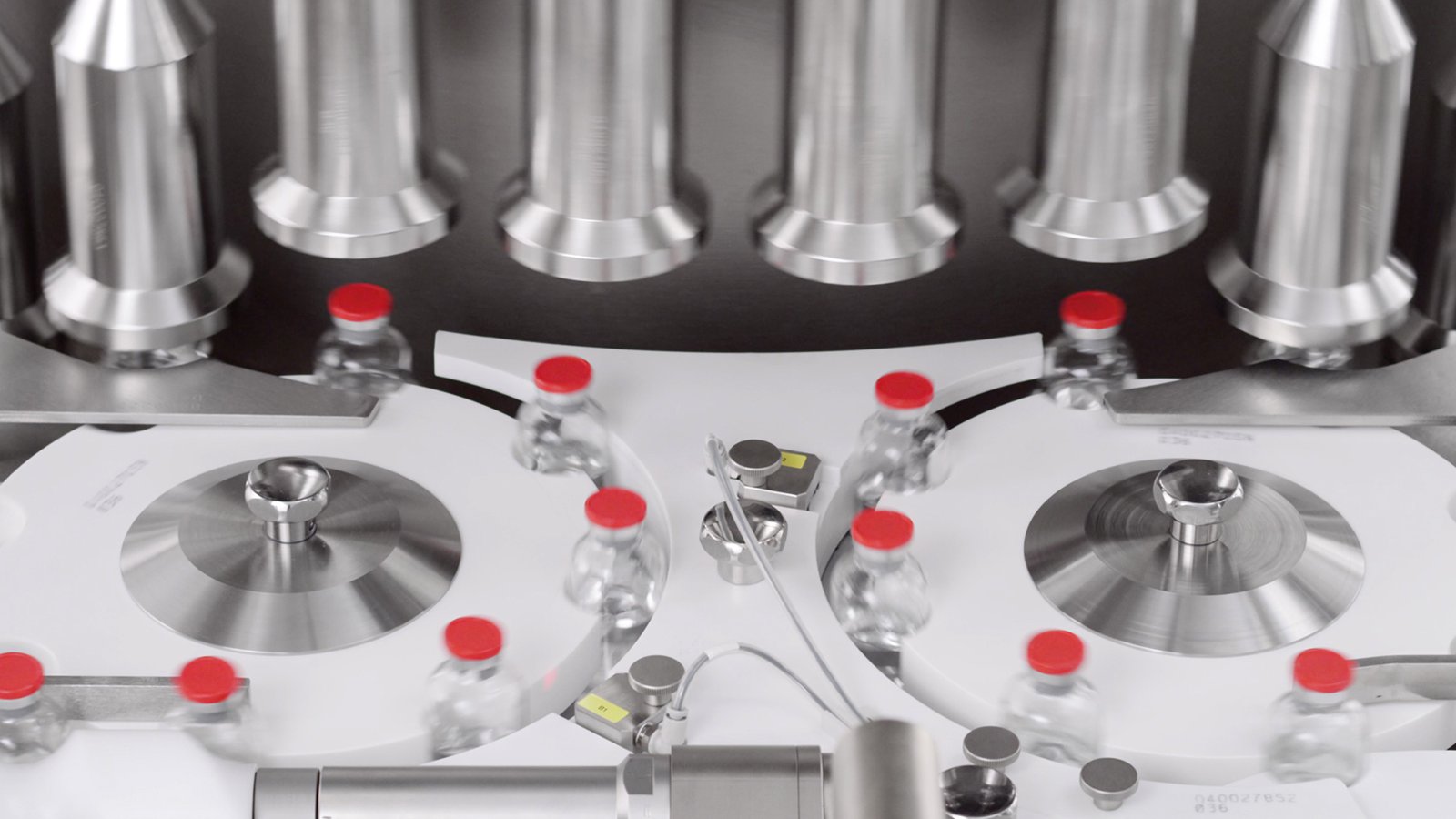
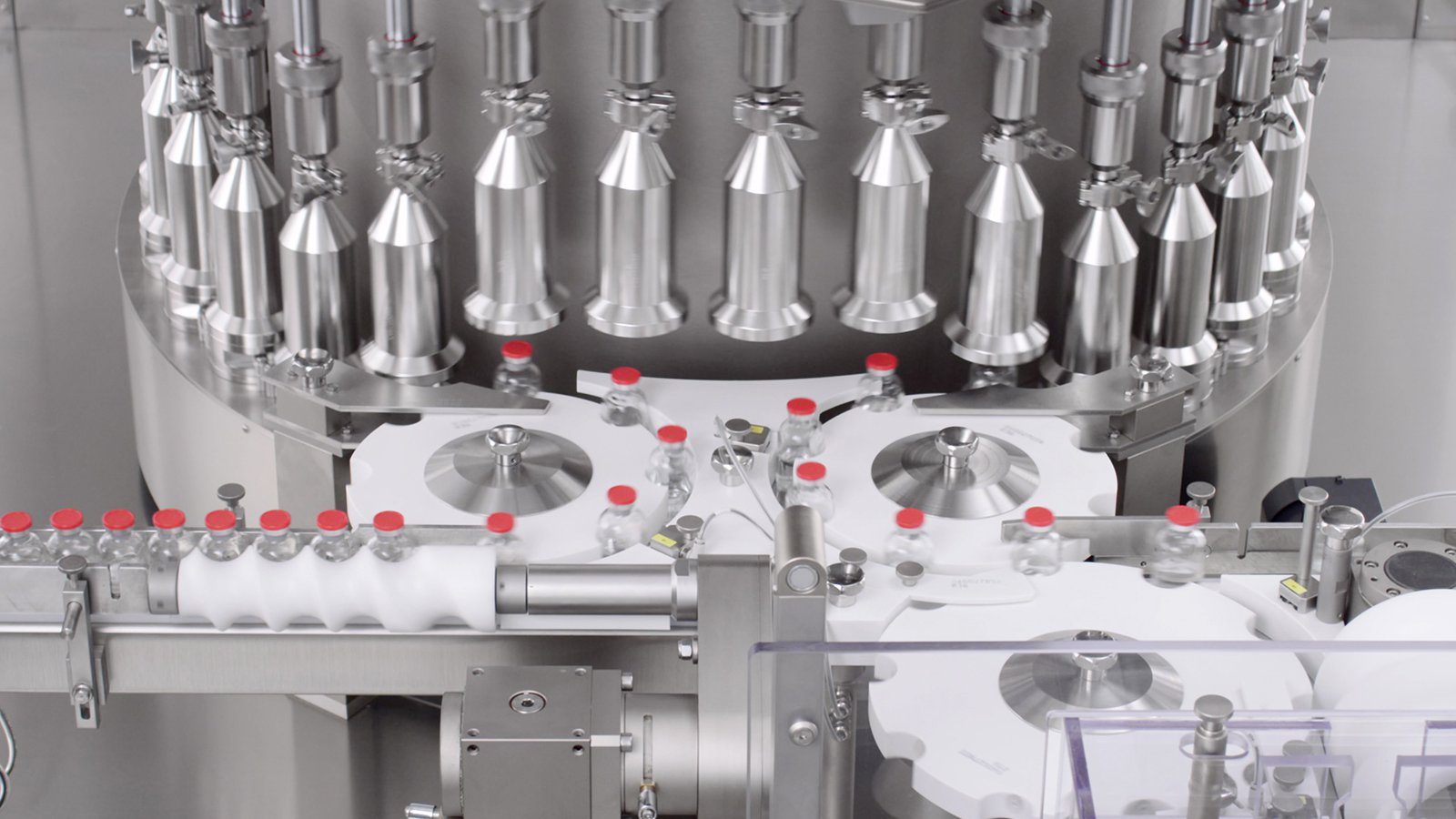
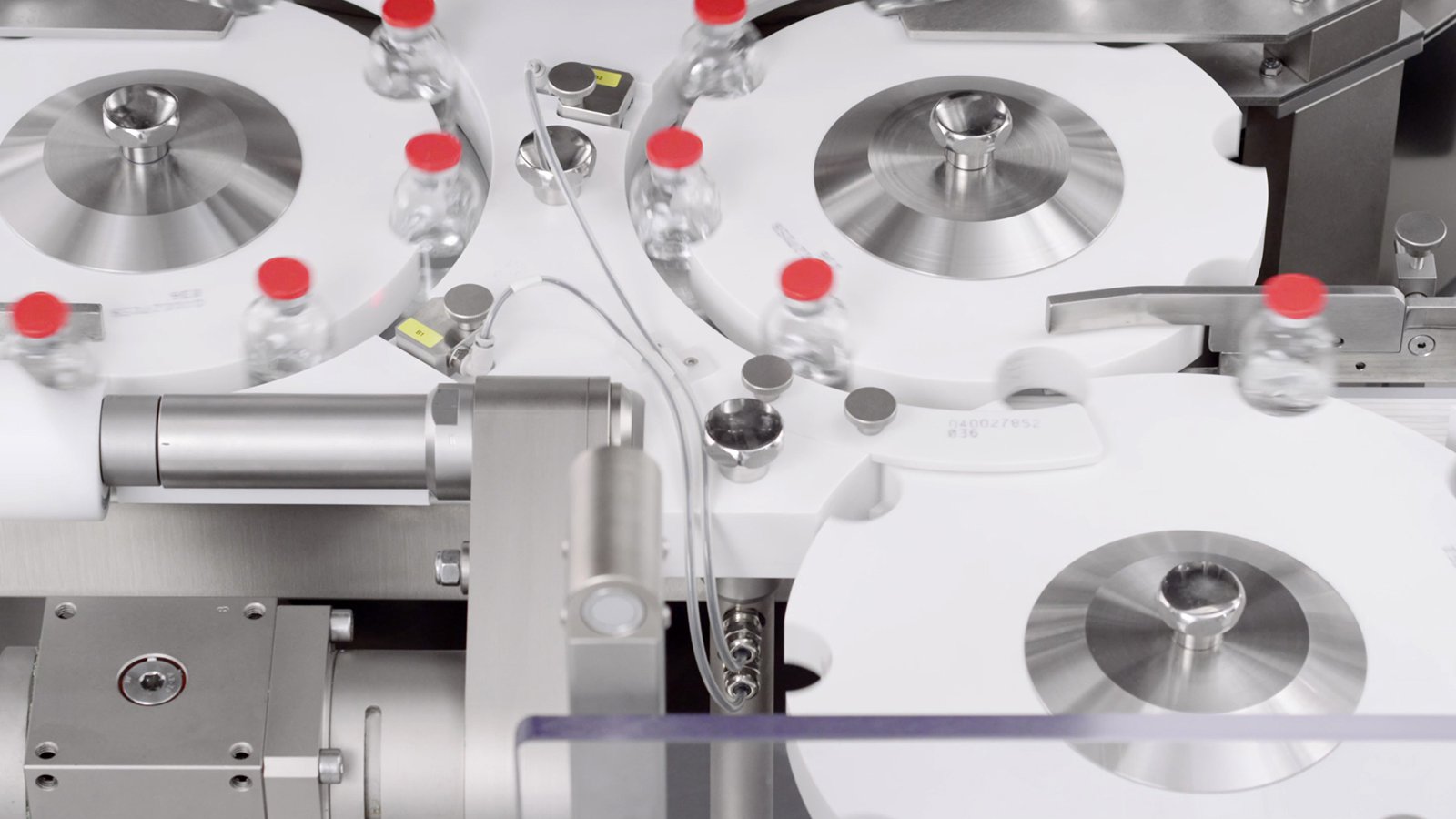
PK-V In-line Vials and Bottles CCI Tester
Pharmaceuticals
Non-Invasive, Non-Destructive, 100% in-line Integrity Inspection at high production speed.
PLAY VIDEO
Bottles
Filled Pharmaceutical Monobloc Aerosol Cans
Vials
Highlights
- Autotest in real time
- Easy to validate
- CFR 21 part 11 compliance and 4.0 full integration
- Quick format changeover
- High installation flexibility
- Automatic Drying System, no testing chamber contamination